The SJD Barcelona Children's Hospital begins a CAR-T ARI clinical trial for children with lymphoblastic leukemia
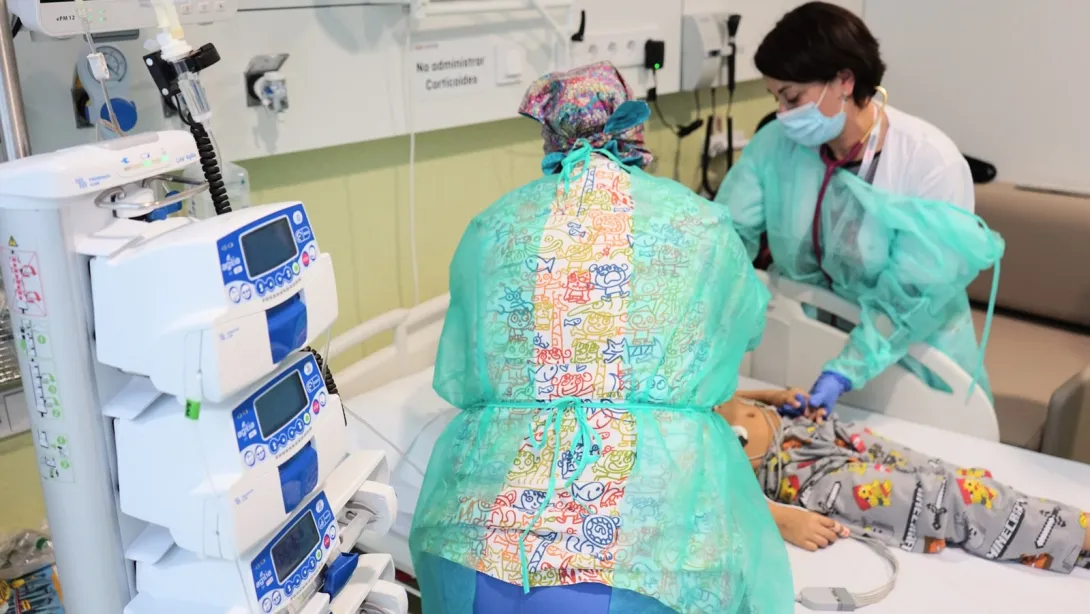
A seven-year-old girl is the first patient to receive this immunotherapy treatment, which is already used in adult patients
This month, the SJD Barcelona Children's Hospital has started a new academic phase clinical trial to assess the efficacy of CAR-T ARI-0001 in pediatric patients. It is developed in partnership with Hospital Clínic, and named in memory of the patient who promoted it and made it possible through a sponsorship campaign. This treatment was approved in 2021 by the Agencia Española del Medicamento y Productos Sanitarios (AEMPS), the Spanish Agency of Medicines and Medical Devices, for hospital use in treating adults over the age of 25 with type-B lymphoblastic leukemia, which is resistant to conventional treatments.
The approval of the academic phase of CAR-T came about thanks to good results obtained in an initial trial of ARI in children and adults with type-B lymphoblastic leukemia, which was carried out by both the SJD Barcelona Children’s Hospital and Hospital Clínic. Although results were good in pediatric patients, it was only approved for use in adult patients because an industrial pharmaceutical CAR-T (Kymriah) had already been approved for children three years prior, in 2018. Now, the CAR-T ARI clinical trial has been opened to pediatric patients in less advanced stages of the disease to find out whether this treatment could be used to substitute others that carry more risk and side-effects, such as a hematopoietic stem cell transplant (also known as a bone marrow transplant).
A 7-year-old girl has been the first patient to receive this immunotherapy treatment within the framework of the clinical trial, which is expected to last two years and have around 30 participants from all over Spain. Susana Rives, manager of the Leukemia and Lymphoma Unit at the SJD Pediatric Cancer Center Barcelona in the SJD Barcelona Children's Hospital, made the announcement this week to coincide with International Clinical Trials Day.
How CAR-T works
CAR-T is a therapy that arms the patient’s own immune system with the tools it needs to recognise, attack and destroy cancerous cells in a targeted way. It involves taking a blood sample from the patient through apheresis—a technique that allows blood components to be separated—to obtain T-lymphocytes, a cell found in the patient’s immune system. These lymphocytes are modified in the lab with genetic engineering techniques to have a receptor on the cell surface that can recognise tumor antigens expressed by type-B leukemias and lymphomas. This allows for targeted destruction of cancerous cells.
The SJD Barcelona Children's Hospital was one of the first hospitals in Europe to start working on CAR-T therapies in children. In 2016, it was the only hospital in Spain that took part in the clinical trial studying the safety and efficacy of a CAR-T developed by the pharmaceutical company Novartis. This was the first pediatric-targeted therapy to be approved by the European Medicines Agency (EMA) and included by the Spanish Ministry of Health in their service offering for treating acute lymphoblastic leukemia in secondary relapse. With the development of the academic phase CAR-T ARI, researchers hope to find a new therapy that can be used in first relapses of the disease.
The CAR-T ARI clinical trial for pediatric patients that has been started now is being carried out within the framework of the pediatric research plan developed by Hospital Clínic and the SJD Pediatric Cancer Centre at the SJD Barcelona Children's Hospital, with the aim of CAR-T being approved in the future by the European Medicines Agency, both for adult and pediatric use.
CAR-T and a vaccine for diffuse brainstem tumor (DIPG)
Professionals from the SJD Pediatric Cancer Center at the SJD Barcelona Children's Hospital are soon to begin examining the viability of combining CAR-T with a vaccine created from the patient’s own dendritic cells, to treat diffuse intrinsic pontine glioma, or DIPG, a pediatric brainstem tumor that currently has no cure.
Between 2016 and 2018, the research team developed and tested a vaccine for this tumor, created using the patient’s own dendritic cells, to train their immune system to attack tumor cells in a targeted manner. “Although we triggered an immune response with the vaccine, this response didn't lead to a significant increase in patient survival rates. We believe that CAR-T can intensify this response, and that’s why we want to study tolerance and efficacy of a combined therapeutic treatment”, explained Andrés Morales, Clinical Director of the SJD Pediatric Cancer Center.
Cleanrooms for developing advanced therapies
The SJD Barcelona Children's Hospital is almost done implementing a platform of four cleanrooms in the SJD Pediatric Cancer Center, where advanced therapeutic treatments can be developed and produced. This is an innovative therapeutic effort involving the use of biological or genetically modified materials in disease treatment. Thanks to this new infrastructure, the Hospital can begin producing CAR-T cell therapies and investigate new treatments, such as gene therapy for primary immunodeficiency in pediatric patients, who, at present, have no efficient treatment option.
These rooms are intended and designed to guarantee an extremely high degree of environmental control, ensuring a sterile environment, and avoiding any risk of contamination during the development of drugs or advanced therapies. The rooms meet the highest quality standards in industrial pharmacology and are approved by the Agencia Española del Medicamento y Productos Sanitarios (AEMPS), the Spanish Agency of Medicines and Medical Devices, to produce these therapies.
“This is particularly important, especially taking into consideration the situation: a pediatric hospital like Sant Joan de Déu, a hospital specialised in pediatric cancer, where countless children with rare diseases are treated. It allows us to make progress with Sant Joan de Déu's translational research model, which aims to apply research results to clinical practice as soon as possible”, says Alessandra Magnani, head of the Advanced Therapies and Immunotherapy Platform at SJD Barcelona Children's Hospital.
The cleanrooms are part of the Pediatric Infrastructures for Viral Therapy project (PIVIT), coordinated by lead researcher Manel Juan, head of the Clínic's Immunology Service and of the joint Immunotherapy Platform of SJD Barcelona Children’s Hospital and Hospital Clínic Barcelona. This project is co-financed by the Generalitat de Catalunya's Departament de Recerca i Universitats (Ministry of Research and Universities). They will provide the Hospital, the SJD Pediatric Cancer Center and the Institut de Recerca Sant Joan de Déu (IRSJD, the SJD Research Institute) with facilities that will fuel research and innovation with the highest standards of investigative excellence.



