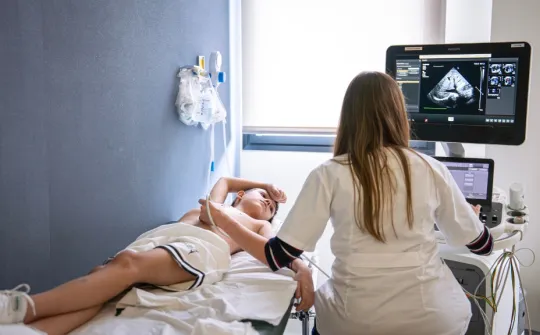
SJD Barcelona Children's Hospital, the first European centre to take part in a clinical trial on a new gene therapy for Duchenne muscular dystrophy
The aim of the therapy is to make up for the lack of dystrophin, an essential protein for muscle development, and slow down the progression of this degenerative disease.
SJD Barcelona Children's Hospital is the first centre in Europe to take part in a clinical trial sponsored by Sarepta Therapeutics in collaboration with Roche of a gene therapy for Duchenne muscular dystrophy. There is currently no cure for this serious degenerative disease. It causes a progressive weakness of the muscles in affected children who, in addition to suffering numerous complications, end up being wheelchair dependent by the age of 12.
Boys with Duchenne muscular dystrophy – it is a disease that only affects boys – cannot produce functional dystrophin protein, an essential protein for maintaining healthy muscles. This is due to a mutation in the gene that codes for this protein, the DMD gene located on the X chromosome.
Now a team of researchers from the pharmaceutical company Sarepta Therapeutics is conducting a gene therapy clinical trial that is aiming to address this lack of dystrophin, and so could slow down the progression of the disease. It consists of administering an intravenous infusion to the patient with a virus modified adeno-associated virus, which contains the genetic sequence that allows muscle cells to produce a smaller but functional dystrophin, microdystrophin.
The preliminary results of the clinical trial carried out in the United States are very encouraging. The patients who received the therapy have not suffered any loss of their abilities in three years and their expression of microdystrophin has increased significantly compared to the group of patients who were in the control group and received placebo. In a second phase of the trial, these patients will also receive the therapy.
120 patients from more than 10 different countries will take part in this trial. The trial shall be open to boys aged from four to seven who are able to walk independently and are in the first phase of the disease. The first patient from the SJD Barcelona Children's Hospital Neuromuscular Diseases Unit to take part in the trial will be a six-year-old boy from Almería (Spain).