A platform to understand COVID-19 in children and pregnancy.
Kids Corona is a research initiative formed to understand whether children have a certain natural protection against COVID-19, and how the disease affects pregnant women. Researchers from around the world, health professionals and the public have joined forces to find answers to these four questions:
- How does COVID-19 impact pregnant women and newborns?
- Do children catch COVID-19?
- How does the disease manifest itself and how did the confinement impact on the children?
- Are children spreaders of the disease?
Next, we collect the answers that have been obtained until the end of the project:
COVID-19 in pregnant women and newborns
Despite the fact that most pregnant women with COVID-19 develop the disease mildly, there are cases of serious presentations. In addition, pregnant women are very vulnerable to the ‘collateral damage’ of COVID-19, that is, to long-term social, economic and welfare consequences.
Research results (updated August 6, 2020)
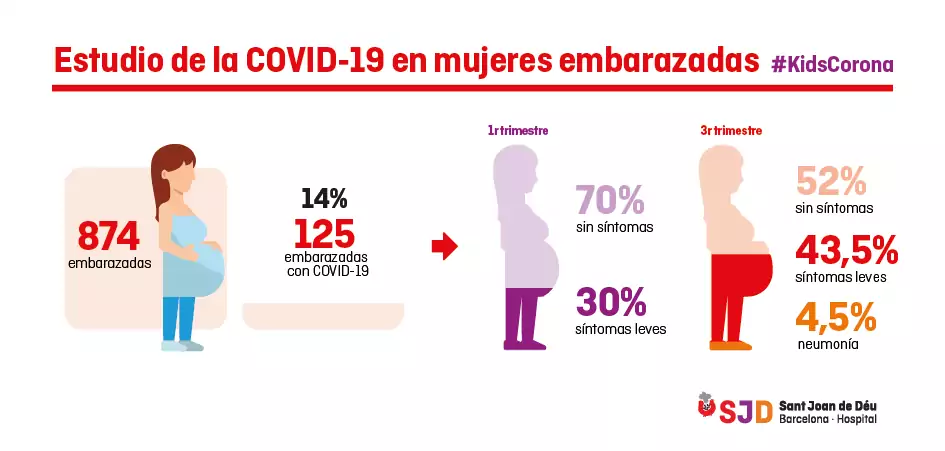
The researchers of this line, carried out within the framework of BCNatal together with Hospital Clínic and which also includes cases from the Hospital de Sant Pau, have been able to analyze 874 cases of pregnant women.
The results show that 14% of pregnant women have antibodies against the coronavirus, and most have developed the infection asymptomatically or with mild symptoms.
The few pregnant women with more severe symptoms are those who contracted the infection during the third trimester of pregnancy (71 women, 52% asymptomatic, 43.5% with mild symptoms and 4.5% with pneumonia), compared with pregnant women with infection during the first trimester (54 women, 70% asymptomatic and 30% with mild symptoms).
These results have been published in The Lancet on August 6, 2020 (Crovetto et al. Seroprevalence and presentation of SARS-CoV-2 in pregnancy).
Infographic of the study in pregnant women
Infection in children
Since the onset of the COVID-19 pandemic, different studies have shown that the disease affects pediatric and adult populations differently.
The number of children affected seems to be much lower than that of adults, despite their being a risk group for serious respiratory infections, and when they become infected, they usually suffer from a milder form of the disease.
Research results (updated June 8, 2020)
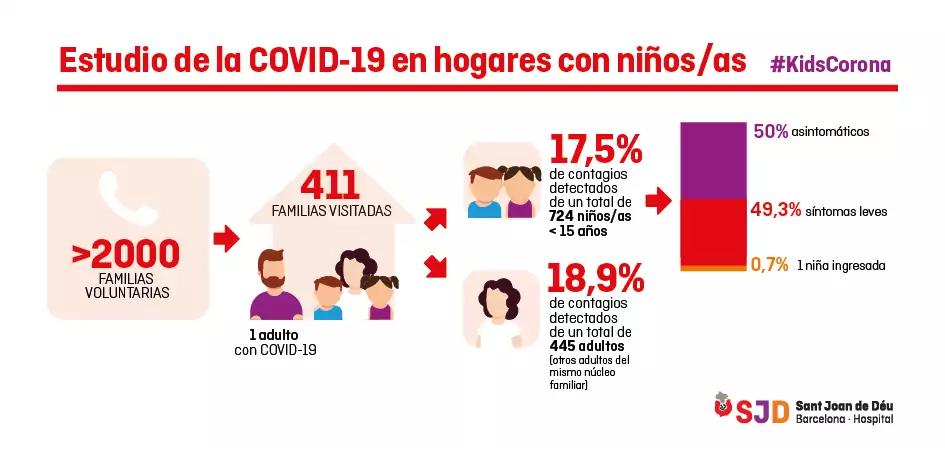
The research team of the Kids Corona platform has visited 411 family homes in which a parent diagnosed with COVID-19 by PCR had been identified.
The preliminary results of the serological tests, in which we take a blood sample to check for antibodies against the coronavirus, showed that 17.5% of the 724 children who lived with a parent who was ill with COVID-19 had also contracted the virus. This is a percentage very similar to that observed in adults who have lived with an infected person (18.9%), so we can conclude that children are infected to a similar extent to adults who come into contact with a case of COVID-19.
However, of the children with COVID-19, more than half had no symptoms and the rest had very mild symptoms, mainly fever, except for one child who required hospital admission.
Therefore, although children become infected with COVID-19 to the same extent as adults, the disease manifests itself in a much milder form in children than in adults.
Study on COVID-19 in homes
Manifestation in children
Research to date has shown that children are generally less likely to develop a severe case of COVID-19, although complicated cases or atypical manifestations of the disease do exist.
An in-depth analysis of the pediatric community, with enough cases and done in a standardized way, is key to assessing the differences in the presentation of COVID-19 compared with adults. Understanding the reason for these differences can help develop treatments and prevention strategies for the entire population.
Aside from the manifestation of the disease itself, a very relevant consequence of the COVID-19 crisis was home confinement. This preventive measure, which was initially taken to protect the physical health of children and other vulnerable groups, has also had effects on their mental health.
During the months of confinement, various stressors coincided that could promote the onset of a mental health problem. In addition, some of these factors have persisted or reappeared in the months following the most restrictive confinement.
What the impact of these stressors on the mental health of children and adolescents has been is a key question we need to answer.
Research results (updated January 22, 2021)
Researchers in the EmCoVID19 study have collected data from 1,529 children and adolescents to assess their risk of developing a mental illness during the COVID-19 lockdown.
Through online questionnaires addressed at the caregivers of young people aged 4 to 18, the researchers looked at whether the emotional and behavioral problems of this group of children and adolescents had increased compared with the previous year. In addition, they also assessed which groups are the most vulnerable and which factors are able to better predict the presence of more emotional and behavioral disorders.
The results show that, in the population of children and adolescents studied, emotional and behavioral problems that could increase the risk of a mental health problem more than doubled during confinement (from 13% to 34.7 %), with children under 8 and a half having a higher risk of suffering such problems. Depression and anxiety were the most prevalent symptoms (64.5%), followed by hyperactivity and behavior change (30.8%). It is worth noting the need for longitudinal data to be able to state that they are probably people at risk of developing a mental health problem, and also that, owing to the tools used, it is only an approximation, not a diagnosis.
The study also found that the factors that best predict the presence of an increased risk of emotional and behavioral disorders are different in children and adolescents. On the one hand, stress and depression in caregivers are the best predictors in children, while in adolescents the best predictor is their style of coping. This suggests that improving the caregiver’s mental state and helping teens cope with stress by focusing on the problem are important goals for preventing mental illness in children and adolescents, respectively. These strategies should be a priority to ensure the mental health of children under conditions of great stress, such as confinement due to COVID-19.
Transmission in children
To answer this question, the SJD Barcelona Children's Hospital has designed several studies to evaluate the infectiousness and transmission of the SARS-CoV-2 coronavirus among the child population in a school-like environment.
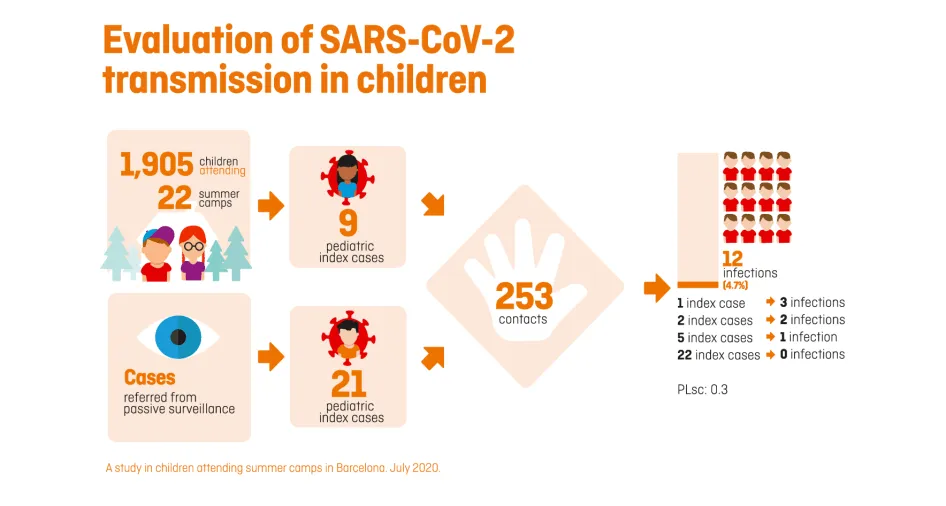
One of the most extensive studies has been that of summer camps, which has systematically collected samples of 1,905 participants in 22 summer camps in the Barcelona area over five weeks, in addition to investigating other coexistence groups from other camps where one of the children or their monitor had been diagnosed with COVID-19. In total, the study has included more than 2,000 participants and is one of the largest that has been carried out worldwide to assess the infectivity and transmission of the SARS-CoV-2 among the child population in a school-like environment, as the summer camps are.
This project is complemented by other studies, such as a collaboration with FC Barcelona, which began in August 2020. It is an investigation with more than 150 child players as well as the staff with whom they live. This study has made it possible to evaluate the transmission of the virus for several months within a school-like environment, in this case during sports activities.
Kids Corona platform’s study of summer camps
Research results (updated August 26, 2020)
The research team of the Kids Corona platform has systematically analysed samples of more than 2,000 participants in summer camps in the Barcelona area for five weeks.
The preliminary results of the PCR tests, in which we take a saliva or nasopharyngeal sample to detect the presence of the coronavirus, have identified a total of 39 index cases (new onset): 30 children and 9 monitors.
The 30 paediatric cases came into contact with 253 children during the camps (belonging to their bubble groups), 12 of which (4.7%) were infected (secondary positives), which represents a local empirical reproduction number (R) of 0.3. This rate is almost six times lower than that of the general population at the time of the study (1.7 to 2) in the areas where the camps were held.
Most of the paediatric index cases (22) detected did not transmit any infection in the camps. Five index cases transmitted the infection to one contact, two to two contacts and one to three contacts. Regarding the ages of the children analysed, it should be stressed that, in this study, the younger children (under 12 years of age) were found to have the same propensity to transmit the disease as the older children (13 to 17 years).
Kids Corona platform’s study of summer camps
The activities took place in a school-like environment, implementing basic risk containment measures: frequent handwashing, small bubble groups, use of a mask and primarily outdoor activities.
The study shows that the distribution of children in bubble groups is effective in containing the transmission of the infection, in facilitating the traceability of contacts and in allowing selective quarantine. In addition, protocolized handwashing five or more times a day has been associated with a decrease in the transmission of the disease.
A high correlation has also been found between the incidence of infection in the general population and the number of index cases detected in camps from the same area, which suggests that participants in summer camps have not been substantial transmitters of the disease and that proactive screening in high incidence areas can be very effective.
Although some limitations of the study must be taken into account, such as the reduced time of the study (five weeks) or the fact that many of the activities of the summer camps have been carried out outdoors and with small groups, meaning that the results cannot be directly extrapolated to other conditions, the data obtained gives hints to open schools in September in a safe and controlled way, applying measures such as those that have been implemented in summer camps: bubble groups, use of masks and frequent handwashing.


